My research interests revolve around label-free optical technologies, focusing on the natural interaction between light and biological samples. It is my goal to find the right optical tools for a given biomedical problem. I have hands-on experience in multiphoton imaging, endomicroscopy, Raman spectroscopy, Diffuse Reflectance Spectroscopy (DRS) and Diffuse Correlation Spectroscopy (DCS). I also utilize computational tools and machine learning to extract meaningful information, for instance by using Digital staining. I enjoy interdisciplinary research and applied those technologies to Inflammatory Bowel Diseases (IBD), muscle tissues, cognitive neuroscience or cancer research.
You can find a complete list of my articles on Google Scholar or on my Research Gate profile. A list of my conference presentations is linked here.
Label-free Multiphoton Microscopy for Inflammatory Bowel Diseases (IBD)
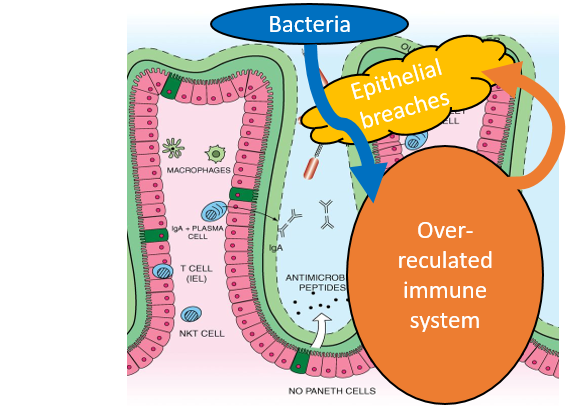
Inflammatory Bowel Diseases (IBD) are triggered by the vicious circle of epithelial tissue damage, infiltration of harmful bacteria and an overly active immune system, which induces further colleratal damage to the tissue, while fighting the bacteria. Together with partners from the Collaborative Research Center “Immune-Epithelial Communication in Inflammatory Bowel Diseases” (TRR241), I investigated many different aspects of this group of diseases.
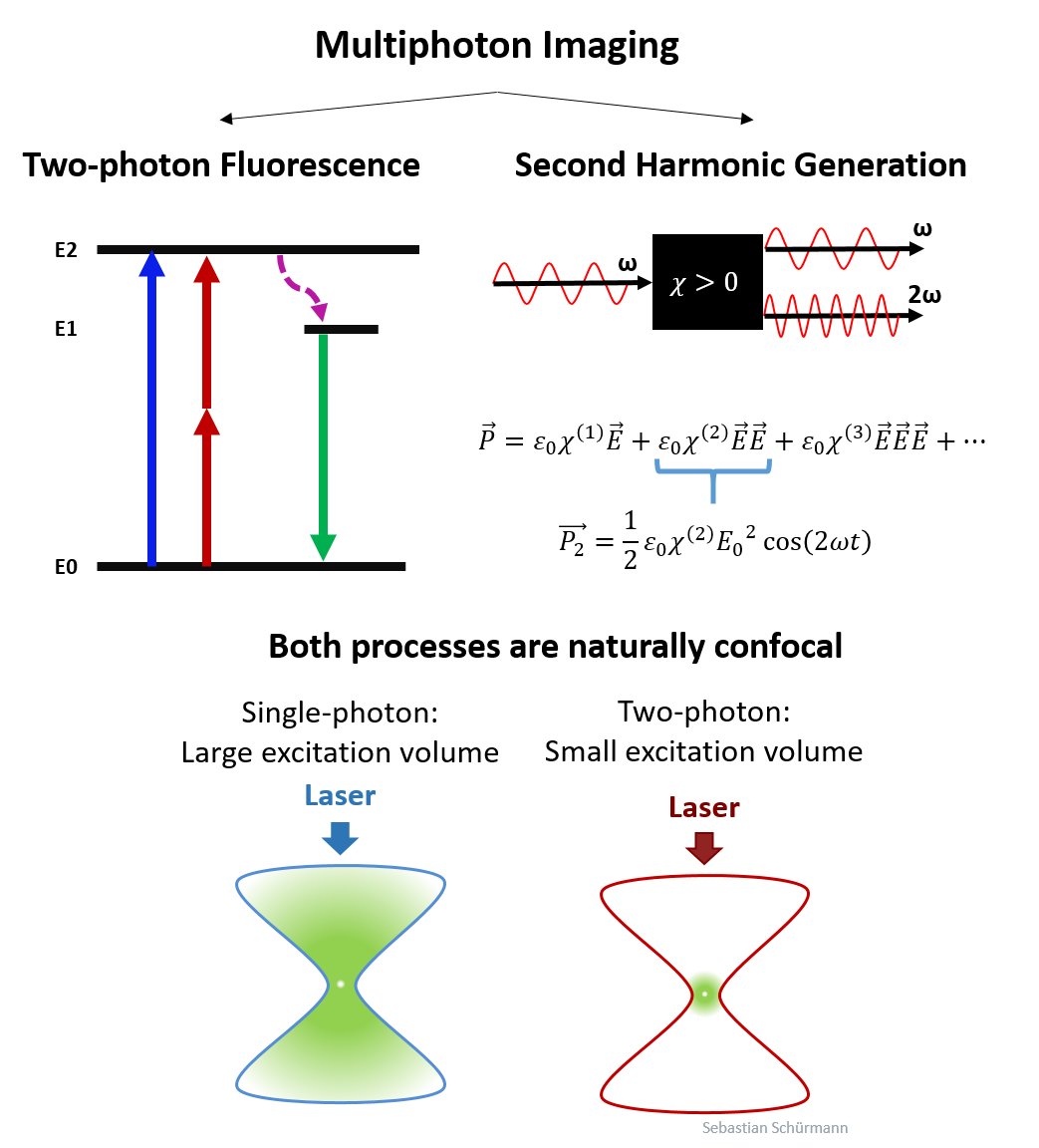
Most often, we used Multiphoton Microscopy to investigate tissue samples. In this technique, high-power ultra-short pulsed lasers are used to excite nonlinear two photon fluorescence (TPF) and Second Harmonic Generation (SHG). Due to their nonlinear nature, both signals are only generated at high photon density, therefore the technique is naturally confocal. The infrared light of the laser ensures a large optical penetration depth. Thus, the technique is ideal for 3D tissue imaging.
Label-free Multiphoton Microscopy uses the signal (TPF and SHG) from natrually occuring molecules, like collagen, NADH and FAD. Thus, staining with artificial markers is not required to image most aspects of the 3D tissue structure. We then speak of autofluorescence, rather than fluorescence, to make the distinction to conventional fluorescence markers.
We have applied this approach to image several different experimental colitis models and identified distinct differences between those models. Read the full publication here. Furthermore, we investigated the autofluorescence signatures in the most important immune cells for IBD. Read that publication here. In collaboration with Angelika SChmalzl from AG Stürtzl, we used it to find bacteria, that express a fluorescent marker in tissue samples with colitis, as you can read here. AG Zundler helped us to find labelled macrophages in mucosal wound healing studies. Besides IBD, we also used MPM to identify tumor growth in an experimental tissue model, together with AG Schneider-Stock, as described here.
Multiphoton ENDO-Microscopy for in vivo imaging of 3D tissue structure
In the team at the Institute of Medical Biotechnology, we developed a new endoscopic system of this Multiphoton Microscope, which we call Multiphoton Endomicroscope (MPEM). The device has a gradient index (GRIN) objetive lens, which acts as rigit endoscope for animals. It has a field of view of 290x290µm², an outer diameter of 1.2 mm and a length of 2 cm. This device allows us to get 3D images of the untreated colon tissue, from a living animal and without acquiring biopsies.
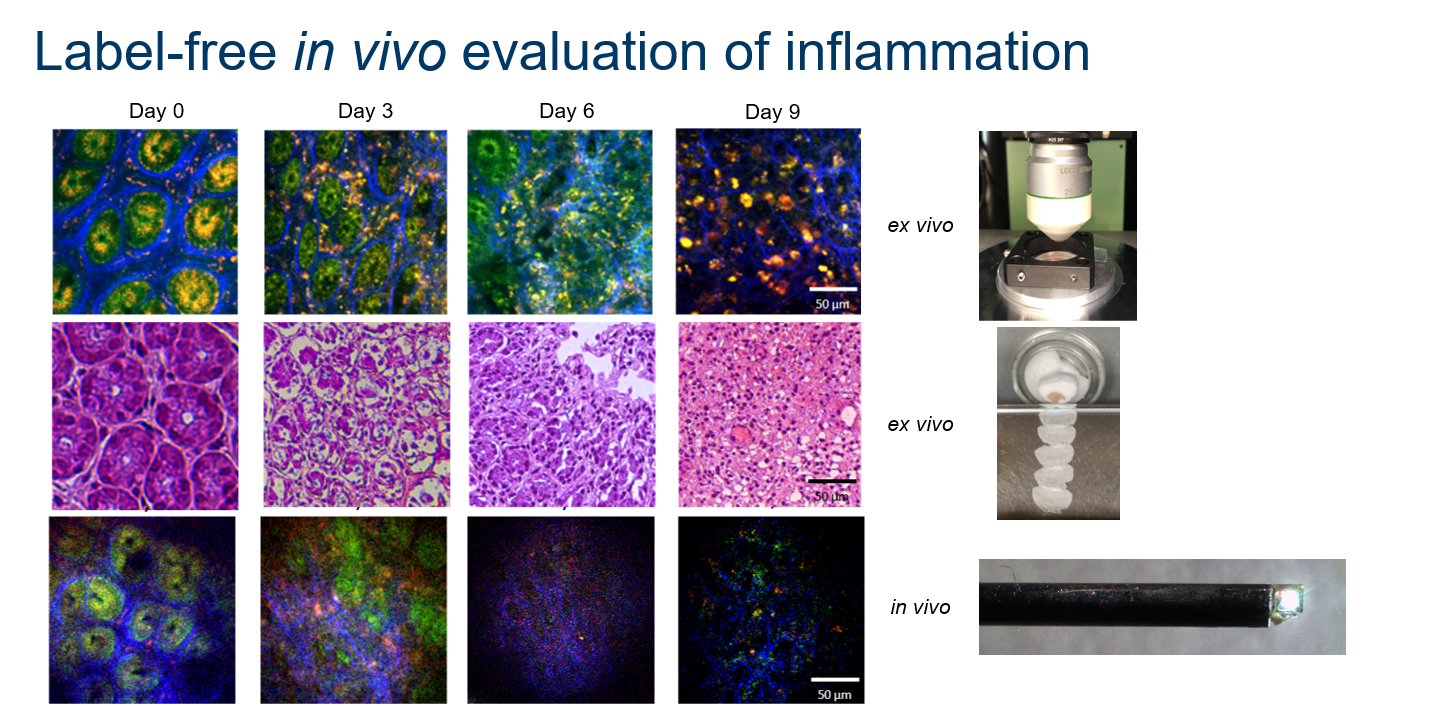
A direct comparision of label-free MPEM images from the living specimen with label-free MPM images and conventional H&E images of acqiuired tissue samples, shows how well the results match. Although the image quality is slighty worse compared to the clean conditions of extracted samples, this system quickly became our working horse.
All technical details about this device are described here. We later upgraded the device by two additional detection channels and applied to two different murine colitis models (see DOI). Currently we are working on experimental studies to use MPEM for finding labelled immune cells in colitis models and to measure leakyness in blood vessels during IBD.
Optical clearing
To increase the optical penetration depth even further, biological specimen can be optically cleared. Thereby, the samples are basically soaked in a medium until the refractive index is more uniform and the sample becomes more transparent. This allows to image the entire colon, from the outside wall until the inside lumen. Using the motion of the sample stage and simple grid-based image stitching, we can then reconstruct large 3D volumes of the entire colon at cellular resolution. Our clearing protocol for Multiphoton Imaging is described here.
CURRENTLY UNDER CONSTRUCTION !
Raman Spectroscopy
DRS and Raman of human hip biopsies: DOI
Identification of reactive species upon treatment with a potential cancer treatment: DOI)
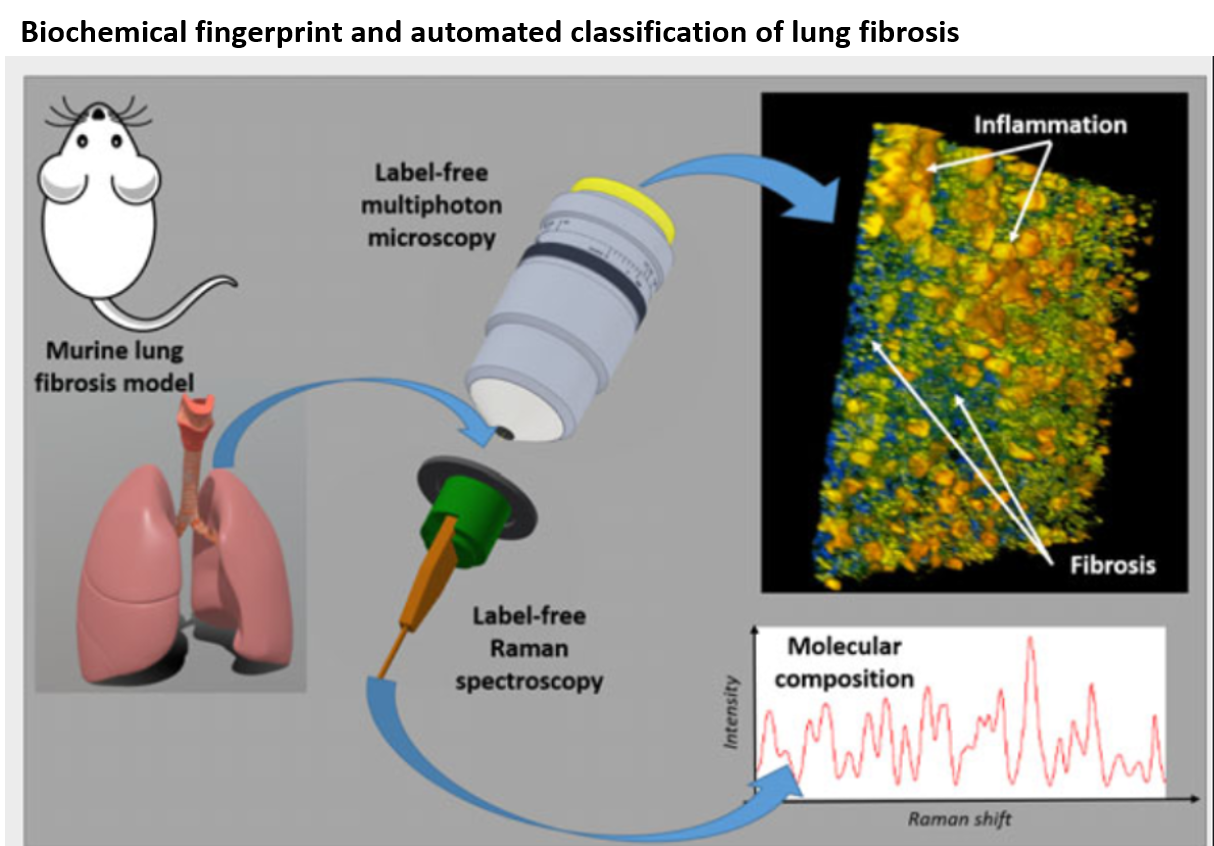
Raman and MPM: DOI
Diffuse Correlation Spectroscopy
I was first introduced to the technique of Diffuse Correlation Spectroscopy (DCS) at the Computational Optics Lab at Duke, in collaboration with Roarke Horstmeyer, Shiqi Xu and Melissa Wu.
Imaging Dynamics Beneath: DOI
Motion classification: DOI

